Does Japan Require Iec-60601 4Th Edition
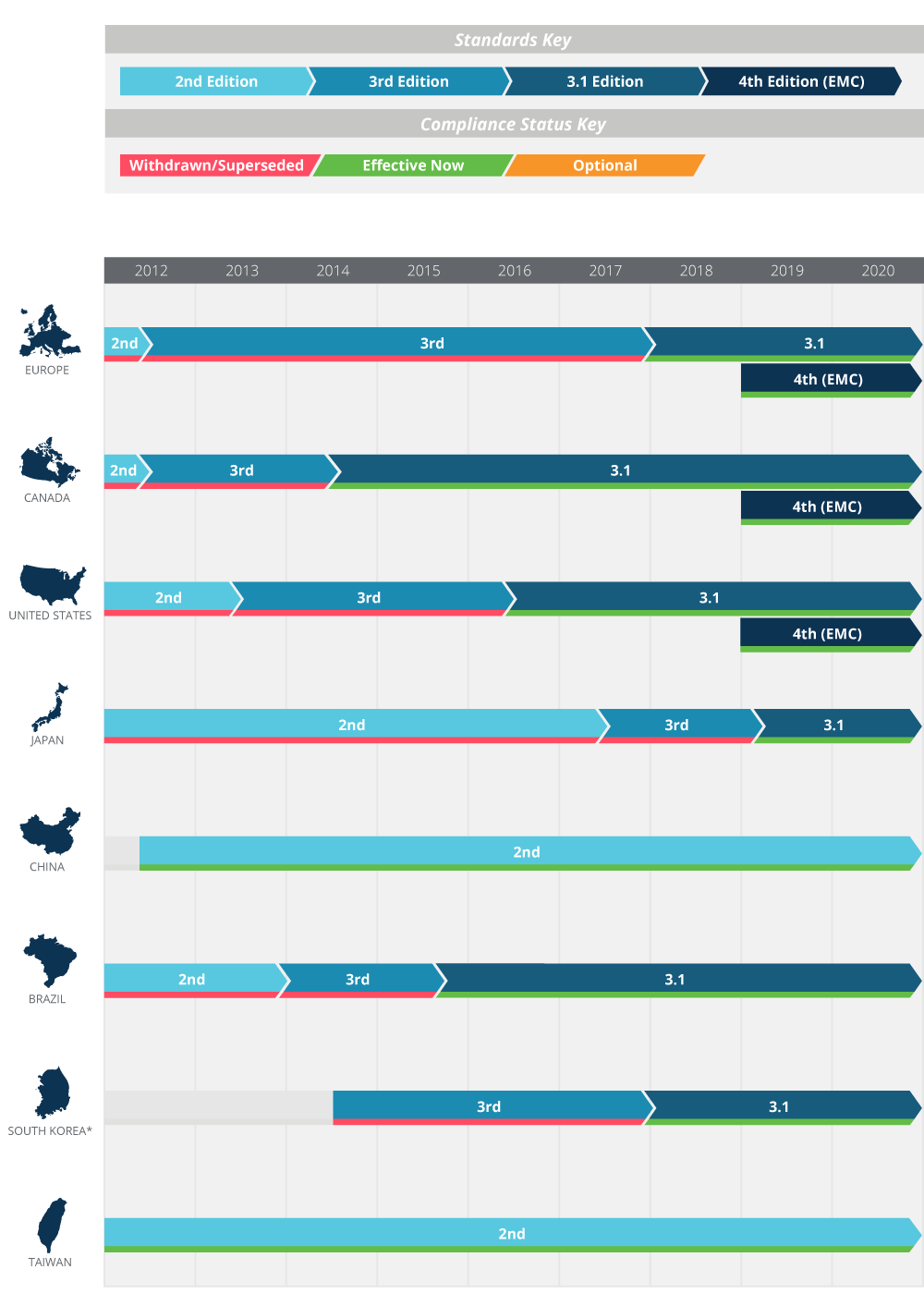
Web Edition 31 introduced in 2012 made over 500 changes and clarifications to the 3rd edition to address ambiguities arising from evolving medical technology. Most recently in 2014 the 4th edition of collateral standard IEC 60601-1-2 Electromagnetic disturbances Requirements and tests was published.
90w Medizinisches Netzteil Konform Zu Iec 60601 1 2 4th Edition 60950 1 Und Ul1310 Nec Klasse 2 Lps Mit 12 48v Ausgangsspannung Globtek
Web IEC 60601 is a series of technical standards for the safety and essential performance of medical electrical equipment published by the International Electrotechnical CommissionFirst published in 1977 and regularly updated and restructured as of 2011 it consists of a general standard about 10 collateral standards and about 80 particular.
. Editions 2 3 and 31.
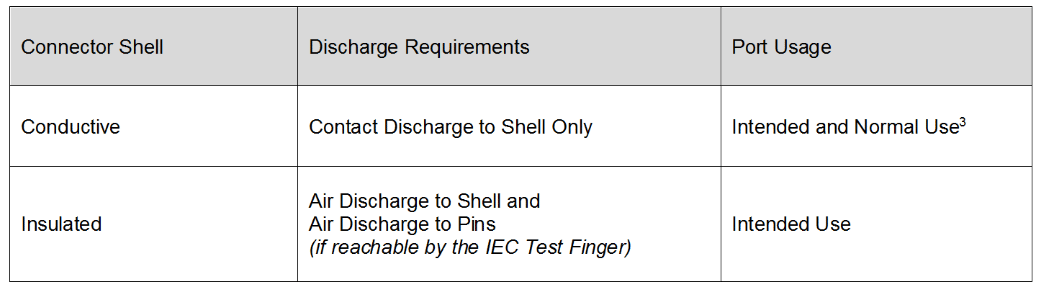
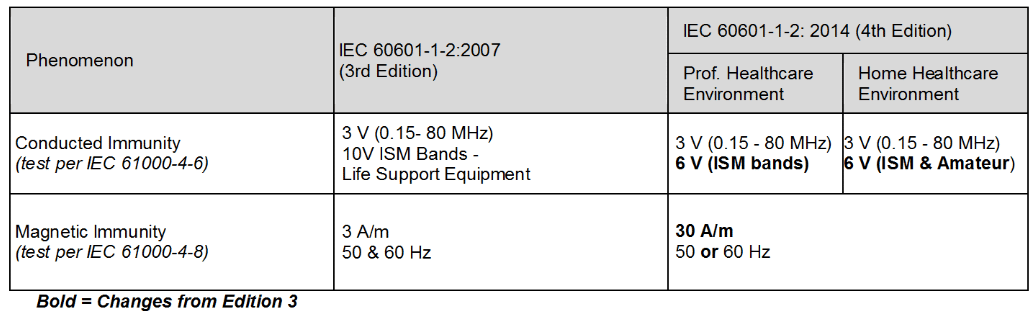
Review Of Iec 60601 1 2 2014 4th Edition Interference Technology

Understanding Medical Power Supply Requirements Avnet Abacus

Be Prepared For The 4th Edition Of The Iec 60601 1 Medical Standard

Webinar On Iec 60601 1 2 Ed 4 1 Ul Solutions

Iec 60601 1 2 4th Edition What You Need To Know Cui Inc

Review Of Iec 60601 1 2 2014 4th Edition Interference Technology

Iec 60601 2 28 International Electrotechnical Commission 60601 Pdf4pro
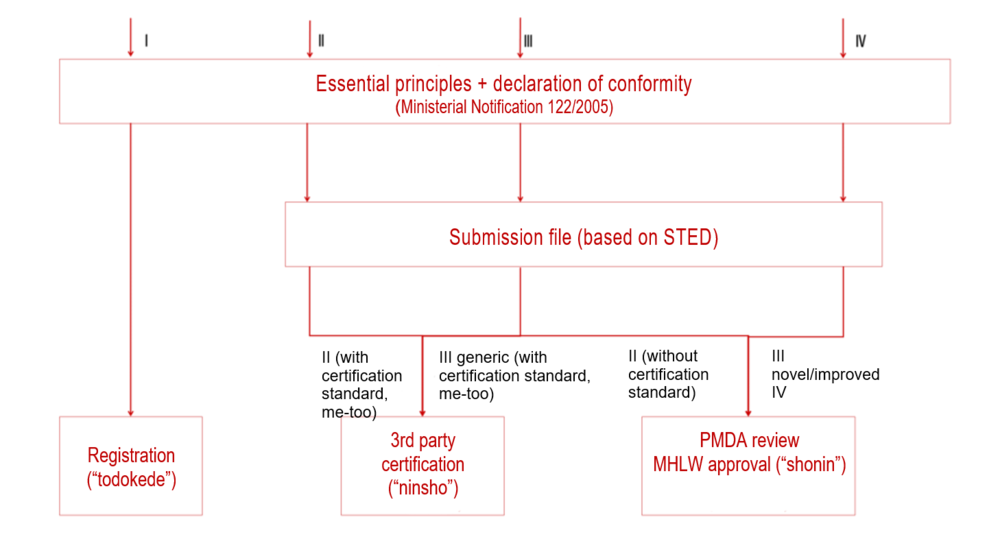
Authorization Of Medical Devices In Japan

Understanding The Impact Of 60601 3rd Edition On Power Design Edn
Power Solutions Designed For Medical Applications Coil Technology Corporation
Iec 60601 1 Medical Design Standards For Power Supplies Cui Inc

Reduced Time Cost China Plans Latest Edition Of Iec 60601 1

Latest Updates On Cdrh Standards Program And Iec 60601
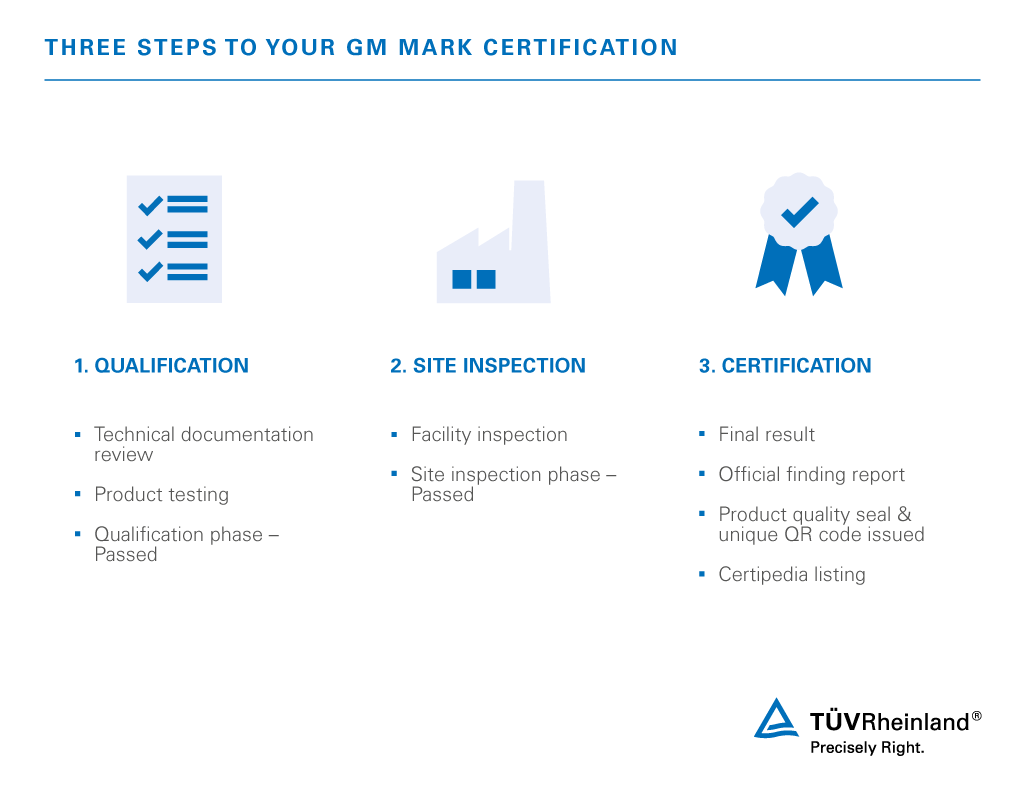
Non Active Medical Device Testing Us Tuv Rheinland
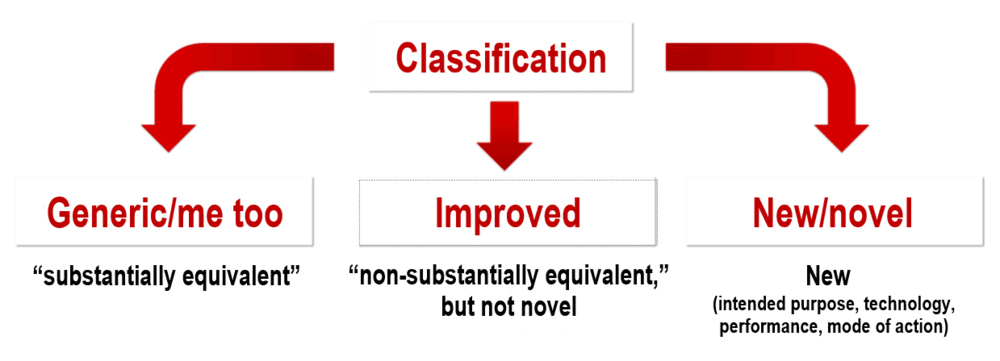
Authorization Of Medical Devices In Japan

The Iec 60601 Amendment Updates Have Published Changes And Impacts In Compliance Magazine

Top 7 Questions About Iec 60601 1 2 4th Edition For Emc Testing Of Medical Devices Eurofins E E North America